Abstract
Background: CD19 negative escape is a major cause of relapse after CD19 CAR T cell therapy for relapsed/refractory (r/r) paediatric Acute Lymphoblastic Leukemia (ALL) and dual targeting of CD19/CD22 may overcome this. We have previously shown that AUTO1, a fast off rate autologous CD19 CAR T cell therapy was highly active in ALL with a favorable safety profile and excellent persistence (Ghorashian et al Nat.Med. 2019). Building on these properties, we developed AUTO1/22 an autologous CAR T cell product co-transduced with two different lentiviral vectors encoding our existing CD19 CAR and a novel CD22CAR designed to recognise targets with low antigen density. We have evaluated the safety and biological efficacy of AUTO1/22 in a Phase I study in children/young adults with r/r ALL (NCT02443831).
Methods: Patients with r/r B-ALL age < 25 yrs who were ineligible for/relapsed after Tisagenlecleucel were recruited. Following fludarabine/cyclophosphamide lymphodepletion, patients received 1x106 /kg CAR+ T cells. The presence of CAR T cells in the blood/bone marrow (BM) was assessed by flow cytometry + qPCR and BM MRD was assessed by IgH qPCR + flow cytometry. Primary endpoints were incidence of grade 3-5 toxicity and the proportion of patients achieving MRD negative remission.
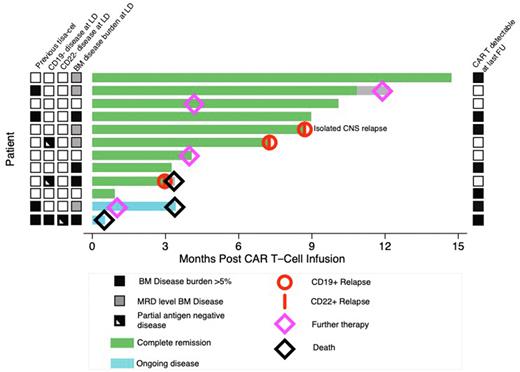
Results: 12 patients have been treated. The median age was 12 years (range 3-21) and patients had a median of 3 prior lines of therapy (range 2-6). Six of 12 patients had relapsed post allogeneic SCT, 6 had received prior Blinatumomab/Inotuzumab and 4 had relapsed after prior Tisagenlecleucel. Prior to lymphodepletion, 4 patients had >5% BM disease by morphology/flow, 5 had detectable BM MRD and 3 were BM MRD negative. Six patients had extramedullary relapse (of which 2 had non-CNS EM disease). Three had detectable CD19 negative disease at enrolment. One of these was completely CD19 negative and in addition had a 5% CD22 negative population.
CAR T cell products had a central memory phenotype with predominance of CD19/22CAR double positive cells (median 54.4%) and balanced populations of CD19 and CD22 single positive cells (13.1% and 11.6% respectively).
Cytokine release syndrome (CRS) occurred in 11/12 patients (grade 1 n=5, grade 2 n=6) requiring Tocilizumab in 5 cases, but severe (≥ grade 3) CRS was not seen and no patients required ICU admission for CRS. Grade 1-2 ICANS was observed in 5 patients. One patient had delayed grade 4 leucoencephalopathy (MRI/brain biopsy were more indicative of fludarabine toxicity than CAR T related) and has ongoing neurological recovery. Nine patients had grade 3-4 cytopenia persisting beyond/recurring after day 28, requiring a CD34+ stem cell top up in 1 case.
Flow cytometry showed significant initial expansion of all 3 CAR +ve populations early post-infusion but CD19/22CAR double positive cells were lost at later time points. Six patients had circulating CAR T cells by qPCR at last follow up and the median duration of B cell aplasia has not been reached. Ten of 12 patients (83%) achieved MRD negative CR/CRi at one month post-infusion and 2 patients did not respond. Importantly 2/3 patients with CD19-ve disease achieved molecular CR demonstrating the efficacy of our CD22CAR. Of the 10 responding patients, 2 have relapsed with CD19+CD22+ disease, one patient with CD19-ve disease pre-infusion has relapsed with CD19partialCD22+ disease and one had emergence of MRD level disease, in all cases associated with loss of CAR T cells. One other patient had early loss of CAR T cells with B cell recovery but ongoing MRD negative CR at 3 months post-infusion and remains in MRD negative CR on maintenance chemotherapy. Overall, at a median follow-up of 8.7 months (range 1-15 months), 6/10 responding patients remain in MRD negative CR at last follow-up. Importantly, antigen-negative relapse has not been observed.
Summary/Conclusion: Our data show that dual CD19/22 targeting CAR T cells generated by co-transduction show a favorable safety profile, with robust expansion/persistence and early efficacy in a heavily pre-treated cohort.
To date with we have not observed antigen negative relapse. This contrasts to an incidence of 5/6 CD19 negative relapses in the 12 responders treated with single CD19 targeting CAR T cells (AUTO1) in an earlier cohort and suggests dual targeting may be effective in preventing antigen escape. However, longer follow up will be needed to confirm this.
Disclosures
Ghorashian:UCLB: Patents & Royalties; Novartis: Honoraria, Speakers Bureau. Yeung:Bristows LLP, 100 Victoria Embankment, London EC4Y 0DH: Consultancy; UCL Business: Patents & Royalties: WO2022064191A1 Named inventor; ADC Therapeutics: Patents & Royalties: WO2022063853A1 Named inventor; Quell Therapeutics: Consultancy. Kokalaki:Autolus Ltd: Current Employment. Pule:Autolus Ltd: Current Employment, Current equity holder in publicly-traded company. Amrolia:Bluebird Bio: Research Funding; Pierre Fabre: Consultancy; UCL Business: Patents & Royalties; Bluebird Bio: Research Funding; Pierre Fabre: Consultancy; Autolus: Patents & Royalties, Research Funding; ADC Therapeutics: Patents & Royalties: named inventor WO2022063853A1.
OffLabel Disclosure:
AUTO1/22 CAR T cells are being investigated and are not currently licensed for therapy of disease
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal